A step-by-step explanation of how to draw the NOF2 Lewis Dot Structure Nitryl fluoride For the NOF2 structure use the periodic table to find the total num. Here are the steps that I follow when drawing a Lewis structure.
The electronic configuration of Nitrogen is 1 s2 2 s2 2 p3.

. Draw nonbonding electrons using the dot notation and bonding electrons as a bond. The NOF Lewis structure is very similar to NOCl and NOBr. Lewis Structure of NaF for counting valence electrons around the terminal sodium atoms.
And just to add that the formal Lewis structure of N O2 is useful when we assess reactivity. The nitrogen atom in N O2 or N 2O4 is said to be quaternized ie. N O2 can READILY dimerize alternatively N 2O4 can readily undergo dissociation.
There are a total of 10 valence electrons in NO. Using the Periodic Table to Draw Lewis Dot Structures. Draw the main Lewis structure of NOF.
N OO OO. N 5 4 - ½ 4 -1. Place one electron pair between each pair of adjacent atoms as determined from the framework found in.
Be sure to put brackets and a positive sign around the NO Lewis structure to show that it is an ion. N 2O4 dinitrogen tetroxide. As an example an oxygen atom has six electrons in its outer shell.
Lewis dot Structure for NaF generated from step-1 and step-2. Draw the main Lewis structure of NOF. The Lewis structure of Nitrogen atom can be drawn if one knows the number of valence electrons of Nitrogen.
Lewis structure for most main group 2-7 compounds can be constructed according to a set of rules. Also there is a -1 charge. Subtracting the number in Step 1 from the number in Step 2 gives you the number of electrons needed to complete the octets.
That will normally be the least electronegative atom C. With NO be sure to remove a valence electron from your total because of the positive sign. Step 2 tells how many electrons are needed and Step 1 is how many electrons you have.
Determine the number of bonding electrons and the number of nonbonding electrons in the structure of BeF2. The nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons. NaF Lewis dot Structure by counting valence electrons on the fluorine atom.
Total number of electrons of the valance shells of nitrogen and oxygen atoms and charge of the anion. Lewis structures are a useful tool for visualizing the electron configuration of compounds. A Lewis structure is based on the concept of the octet rule in which atoms share electrons so that each atom has eight electrons in its outer shell.
Decide which is the central atom in the structure. For cations subtract a number of electrons equal to the positive charge. O 6 4 - ½ 4 0.
Nitrogen atom has 5 valence electrons so its Lewis dot symbol for N is. Identify the total number of valence electrons in the diagram. The best Lewis structure is one that has the fewest formal charges the top structure.
Covalent bonds are formed when one electron from each atom forms an electron pair. Thus the Lewis structure of NO is. A step-by-step explanation of how to draw the NOF Lewis Dot Structure Nitrosyl fluorideFor the NOF structure use the periodic table to find the total numb.
O 6 - 3 ½ 4 1. Drawing the Lewis Structure for NO. There are one nitrogen atom and two oxygen atoms in the nitrate ion.
Enter the number of bonding electrons followed by the number of nonbonding electrons separated by a comma in the dot. Enter the number of bonding electrons followed by the number of nonbonding electrons separated by a comma in the dot. Draw a skeleton structure in which the other atoms are single-bonded to the central atom.
The first step is to figure out how many electrons your diagram should have. Drawing the Lewis Structure for NOF. Draw nonbonding electrons using the dot notation and bonding electrons as a bond.
Nitrogen and oxygen are located at VA and VIA groups respectively in the. N OO. A Lewis Dot Structure can be made for a single atom a covalent compound or a polyatomic ion.
Drawing correct lewis structure is important to draw resonance structures. N 5 - 3 - ½ 4 0. Determine the number of bonding electrons and the number of nonbonding electrons in the structure of BeF2.
A Lewis Dot Structure is drawn by a series of dots lines and atomic symbols and provides a structure for the way that the atom or molecule is arranged. In the NOF Lewis structure Nitrogen N is the least electronegative atom and goes in the center of the Lewis structure. To sketch the NaF Lewis structure by following these instructions.
For anions add a number of electrons equal to the negative charge. Determine the Number of Bonds in the Molecule. Check the formal charges to be sure that each atom has a formal charge of zero.
In a Lewis structure these six dots are arranged so that an atom has two lone pairs and two single electrons.

Draw The Lewis Structure Of Nof Nitrosyl Fluoride Youtube

Nof Lewis Structure How To Draw The Lewis Structure For Nof Youtube
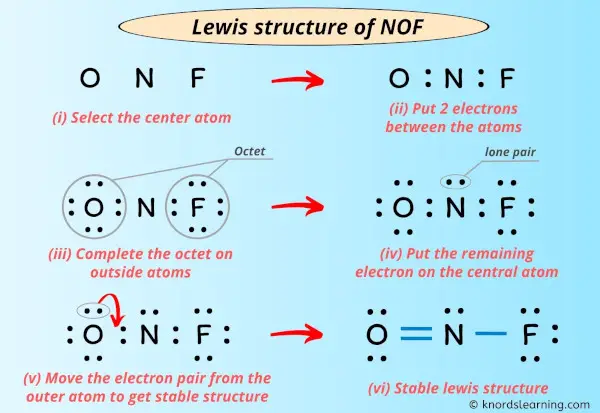
Lewis Structure Of Nof With 6 Simple Steps To Draw
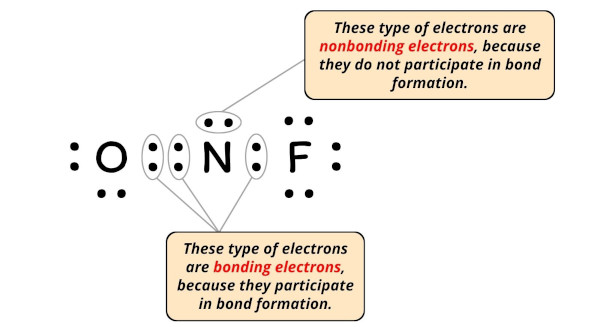
Lewis Structure Of Nof With 6 Simple Steps To Draw

Free Pdf Notes Organic Chemistry Basics Chemistry Basics Organic Chemistry Chemistry

Nof Lewis Structure Geometry Hybridization And Polarity Techiescientist
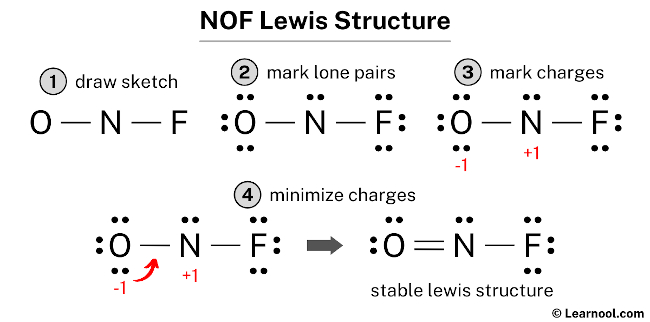
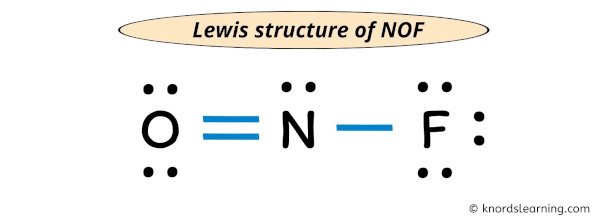

0 comments
Post a Comment